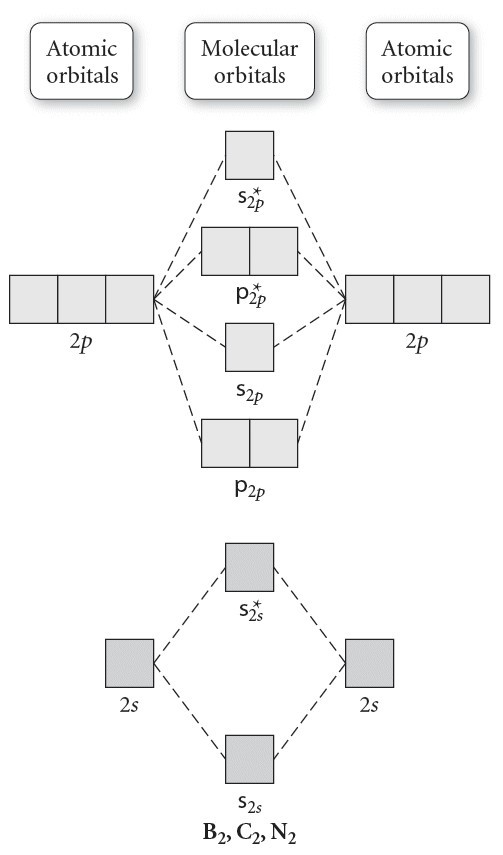
p2 molecular orbital diagram
F has two electrons in the 2s energy level and five electrons in the 2p orbitals. Orbitals exhibit Cs symmetry.

Optimized Molecular Geometry And The Frontier Orbital Density Download Scientific Diagram
Draw the Molecular Orbital Diagram for P2 P2 P2-.

. Draw the Molecular Orbital Diagram for P2 P2 P2-. Molecular Orbital Diagram of O 2 Chapter 9 Section 6 When filling the MO levels you have to. Molecular Orbitals of the Second Energy Level.
Draw the Molecular Orbital Diagram for P2 P2 P2-. This is called quantum jump. Rank these in terms of increasing bond strength.
Up to 3 cash back p2 - Free download as PDF File pdf Text File txt or read online for free. Draw the Molecular Orbital Diagram for P2 P2 P2-. Determine the bond order.
The latter do not possess C2 rotation axes coincident to the infinite-fold rotation axis of the orbitals on the basis of the change in wave function sign upon crossing. Determine the bond order. Atomic orbitals must have the proper symmetry and energy.
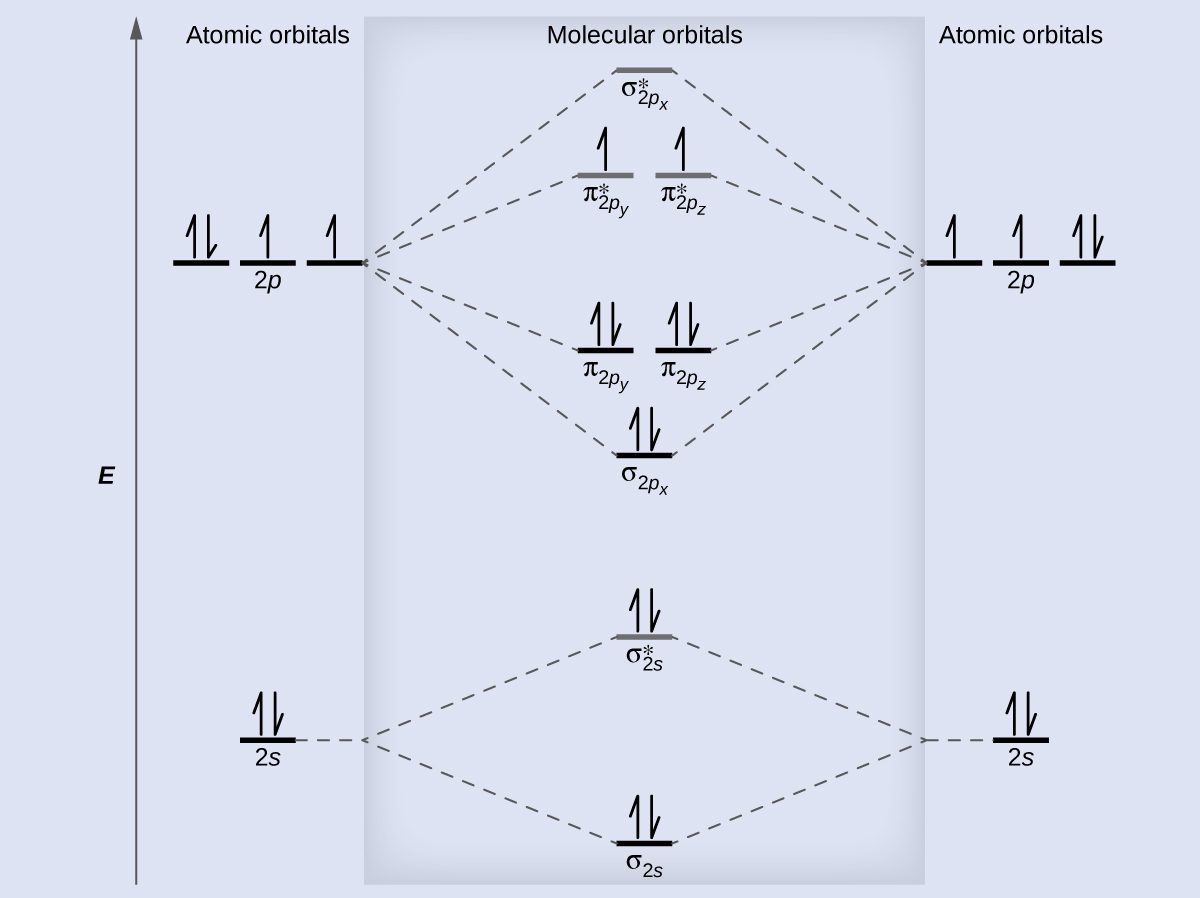
Lets look at the molecular orbital diagram for N 2. Since Nitrogen is a period 2 element we will start the molecular orbital diagram with. To do that we need to find the number.
This problem has been solved. Bond order in a molecule was defined by Linus Pauling as. Scribd is the worlds largest social reading and publishing site.
The head-to-head overlap giving σ molecular orbitals results in greater overlap making its bonding molecular orbital the most stable and lowest energy while the σ antibonding is least. For example B has two electrons in the 2s orbital and one in the 2p orbital. Orbital Diagram of All Elements Diagrams.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. Use evidence to support your answer. Step 3 Fill in the electrons in the.
Rank these in terms of increasing bond strength. Nitrogen has an electron configuration of 1s 2 2s 2 2p 3. Use evidence to support your answer.
The relative energy levels of atomic and molecular. To do that we need to find the number. Count the number of valence electrons Start with the lower energy orbitals first Follow Hunds.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. Bond order in a molecule was defined by Linus Pauling as the difference between the number of bonding electrons and the number of anti-bonding electrons as per Molecular. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s antibonding molecular orbital just like the.
To write the orbital diagram for the Chlorine atom Cl first we need to write the electron configuration for just Cl.

Molecular Orbital Diagram Wikipedia
Solved I Have The Correct Answer But Do Not Understand How Chegg Com

Neet Ug Sigma Molecular Orbital And Pie Molecular Orbital In Hindi Offered By Unacademy

4 10 Second Row Diatomic Molecules Chemistry Libretexts
Using Molecular Orbital Theory Explain Why The Removal Of One Electron In O2 Strengthens Bonding While The Removal Of One Electron In N2 Weakens Bonding Quora

Stabilizing P P P22 P2 And P20 As Bridging Ligands Sciencedirect
M9q5 6 Molecular Orbital Theory Chem 103 104 Resource Book

Chemistry Molecular Structure 32 Of 45 S P2 Hybridization Boron Trifloride Bf3 Youtube

Four Coordinate 14 Electron Ruii Complexes Unusual Trigonal Pyramidal Geometry Enforced By Bis Phosphino Silyl Ligation Journal Of The American Chemical Society

Molecular Orbital Diagram Wikipedia

Solution Tybsc Sem V P2 Unit 1 Test Mot Studypool

Molecular Orbital Diagram Wikipedia
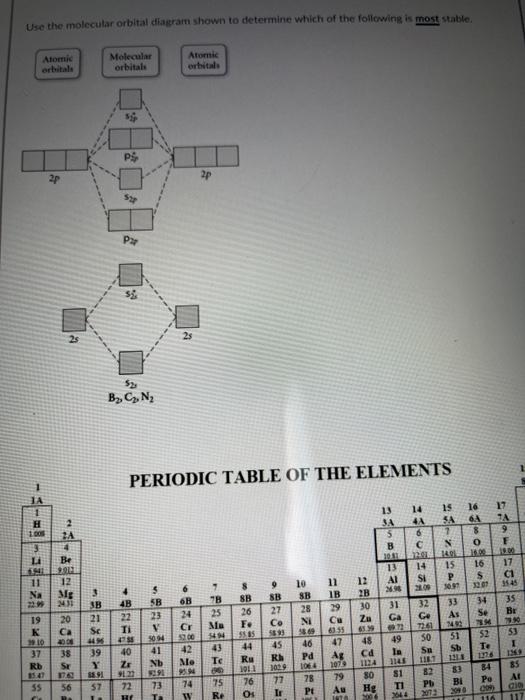
Solved Use The Molecular Orbital Diagram Shown To Determine Chegg Com

Molecular Orbital Diagram Wikipedia
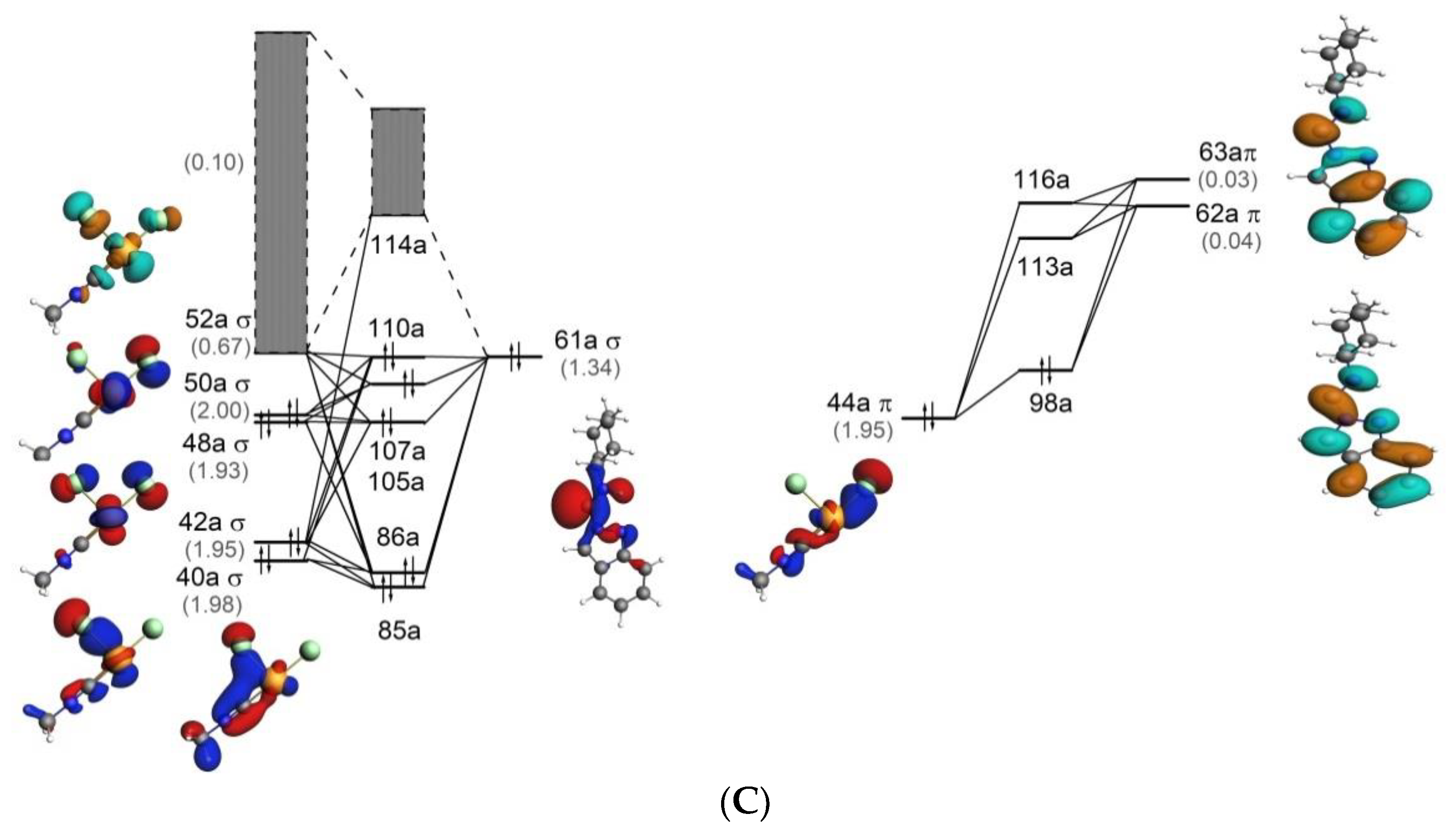
Molecules Free Full Text Reaction Between Indazole And Pd Bound Isocyanides A Theoretical Mechanistic Study Html

Molecular Orbital Mo Diagram For O2 2 Youtube

Stabilizing P P P22 P2 And P20 As Bridging Ligands Sciencedirect