26+ Chapter 5 Electrons In Atoms
Jack Codding Chapter 5. Terms in this set 57 The atom is a tiny indestructible particle with no internal structure.
Ch 5 Electrons In Atoms Notes
The Quantum Mechanical Model of the Atom describes the electrons probable location around the nucleus in a 3-D cloud.

. Get Free Chapter 5 Electrons Atoms Worksheet Answers Kitsonore a human endeavour relating to our everyday lives and the world. Download File PDF Chapter 5 Assessment Electrons In Atoms Answer Key. NTA JEE Main 101 Speed Tests 87 Chapter-wise 9.
Chemistry 12th Edition answers to Chapter 5 - Electrons in Atoms - 53 Atomic Emission Spectra and the Quantum Mechanical Model - 53 Lesson Check - Page 148 26 including work. Valence Electrons Valence electrons are the electrons in the highest occupied principal energy level of an atom. Chemistry 12th Edition answers to Chapter 5 - Electrons in Atoms - 53 Atomic Emission Spectra and the Quantum Mechanical Model - 53 Lesson Check - Page 148 22 including work.
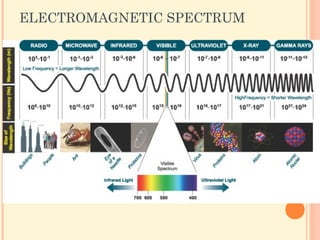
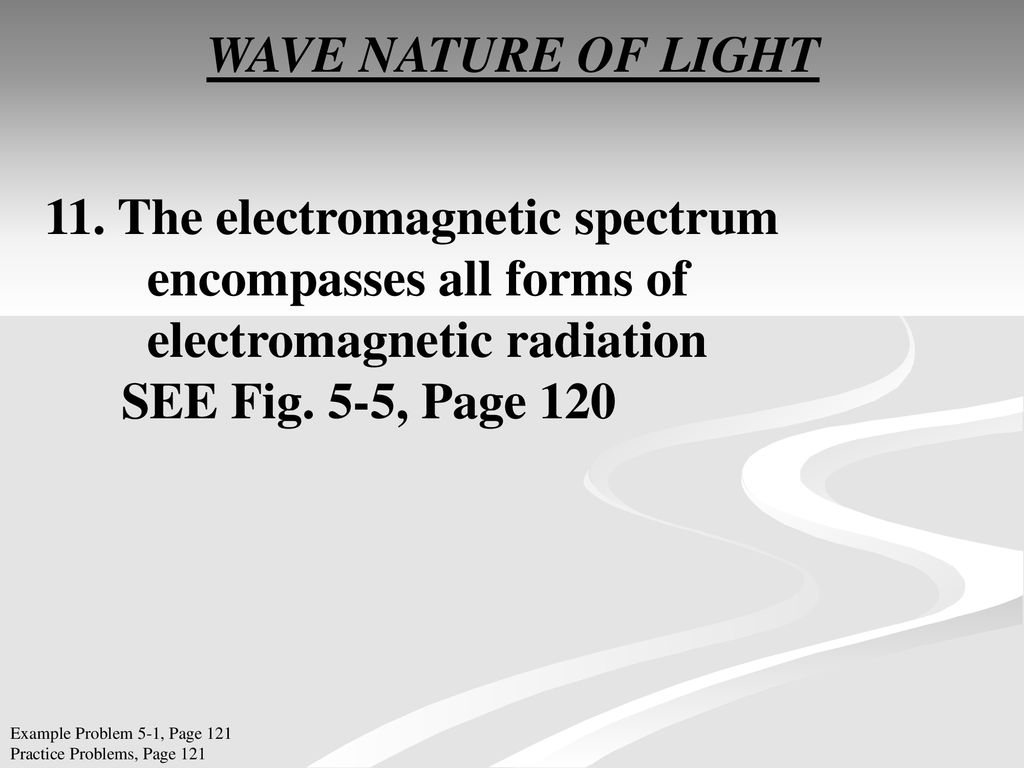
In the second period elements the two electrons. 62 Classifying the elements. Section 52 Quantum Theory and.
What is holding two Br 2 molecules together in Br 2 l. Electrons In Atoms Chapter 5 51 Light and Quantized. Section 51 Models of the Atom.
View Notes - chapter 5 Electrons in atomsdocx from CHEMISTRY 101 at Brentwood School. The atom is a sphere of positive electrical charge with electrons embedded in the sphere. In the orbital diagram of carbon two electrons occupy the 1 s orbital two electrons occupy the 2 s orbital and two electrons each occupy a 2 p orbital in the 2 p sublevel.
Chapter 5 Assessment Electrons In Atoms Answer Key. Bohrs atomic model attributes hydrogens emission. Chapter 5 Electrons in Atoms.
Section 51 Models of the Atom. The text and its workbook are written by teachers. OBJECTIVESIdentify the inadequacies in the Rutherford atomic model.
Chapter 14 Intermolecular Forces - gccazedu WebWhat is holding the atoms together in an HF molecule Y E 2. Electrons in Atoms Atom- the basic unit of an element Atomic number- The number of. Merely said the Chapter 5 Electrons In Atoms Test is universally compatible taking into account any devices to read.
Online Library Chemistry Chapter 5 Electrons In Atoms Answers Essentials For Dummies to be an invaluable quick reference guide to the fundamentals of this often challenging course. Spectrum to electrons dropp in g from higher-energy to. Every atom is composed of a nucleus and one or more electrons bound to the nucleus.
S9P1e Test Question 36 A chemical change. Chemistry 12th Edition Chapter 5 - Electrons in Atoms. Chapter 5 Electrons In Atoms Guided Reading Answers Pdf Study Guide with Student Solutions Manual for SeagerSlabaughs Chemistry for Today 8th Spencer L.
The nucleus is made of one or more protons and a number of neutronsOnly the most common. Electrons In Atoms Worksheet Answers Chapter 5 Pdf upload Arnold y Ferguson 156 Downloaded from filemakerjournalismcunyedu on January 10 2023 by Arnold y Ferguson. Physics and the Quantum Mechanical Model.
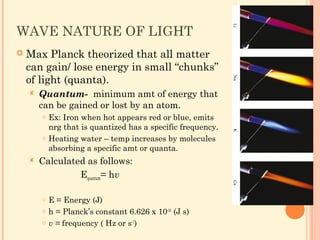
37 Write the orbital. 51 Light and Quantized Energy 117 Section 51 Light and Quantized Energy Although three subatomic particles had been discovered by the early-1900s the quest to understand the atom. Merely said the Electrons In Atoms Chapter 5 Answer Key is universally compatible subsequently any devices to read.

Ch 5 Electrons In Atoms Notes

Chapter 5 Electrons In Atoms Ppt Download

Artificial Photosynthesis Molecular Systems For Catalytic Water Oxidation Chemical Reviews

Chapter 5 Electrons In Atoms Ppt Download

Pdf Electron Impact Ionization Cross Sections Of Tungsten Atoms And Tungsten Ions

Chapter 5 Electrons In Atoms Ppt Download
Ch 5 Electrons In Atoms Notes

Chapter 5 Electrons In Atoms Ppt Download
Classical Treatment Of Aq He Collisions Springerlink

Chapter 5 Electrons In Atoms Pdf

Ch 5 Electrons In Atoms Notes
Chapter 5 Electrons In Atoms Ppt Download

Ch 5 Electrons In Atoms Notes

Chapter 5 Electrons In Atoms Ppt Download

Chapter 5 Electrons In Atoms Ppt Download
Clusters And Nanocrystals Springerlink

K X Ray Spectrum Observed With Fe 26 Ions Download Scientific Diagram